Strengthening Program for Pharmaceutical Startup Ecosystem
Study Group for Encouraging Dialogue between Biotech Venture Businesses and Investors
Examples of initiative to support startup businesses
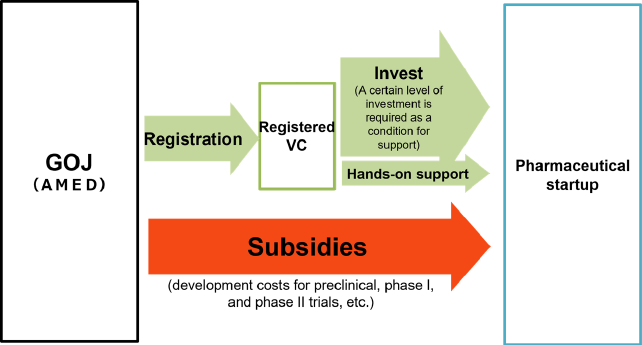
Strengthening Program for Pharmaceutical Startup Ecosystem
A majority of new drugs in recent years have been developed by pharmaceutical startup companies as in the case where those companies were the first to develop vaccines successfully in the face of the current pandemic caused by the Covid-19. Despite the need of substantial funding for the development of new drugs, the existing pharmaceutical startup ecosystem in Japan is not designed to secure necessary funds smoothly when compared with the situations in Europe and the United States.
In order to alleviate the supply shortage of large-scale development funds, this program supports pharmaceutical startup companies on their development aimed at practical application, especially focusing on those engaged in preclinical development, Phase 1 clinical trial or Phase 2 clinical trial. To qualify for this program, funding is required from certified venture capitals specializing in drug development and providing the hands-on commercialization support.
Details of this program and the information regarding the application can be found on the website of Japan Agency for Medical Research and Development (AMED).
Study Group for Encouraging Dialogue between Biotech Venture Businesses and Investors
The "Study Group for Encouraging Dialogue between Biotech Venture Businesses and Investors" was established in 2017 for the purpose of discussing fundraising challenges primarily faced by the pharmaceutical startups immediately before or after becoming listed. The study group has been engaged in identifying issues and discussing optimal policies. Documents from the previous discussions can be viewed here.
◆Ito Review 2.0 : Biomedical Edition (Report of the Study Group for Encouraging Dialogue between Biotech Venture Businesses and Investors)
The study group compiled "Ito Review 2.0 : Biomedical Edition" in April 2018 to summarize the outcome of identifying issues and discussing optimal policies at the meeting of the "Study Group for Encouraging Dialogue between Biotech Venture Businesses and Investors". The report was revised in July 2019 based on the discussions held at the subsequent meeting. Details can be viewed here.
Ito Review 2.0 : Biomedical Edition (Revised Version) (PDF Format :7,214KB)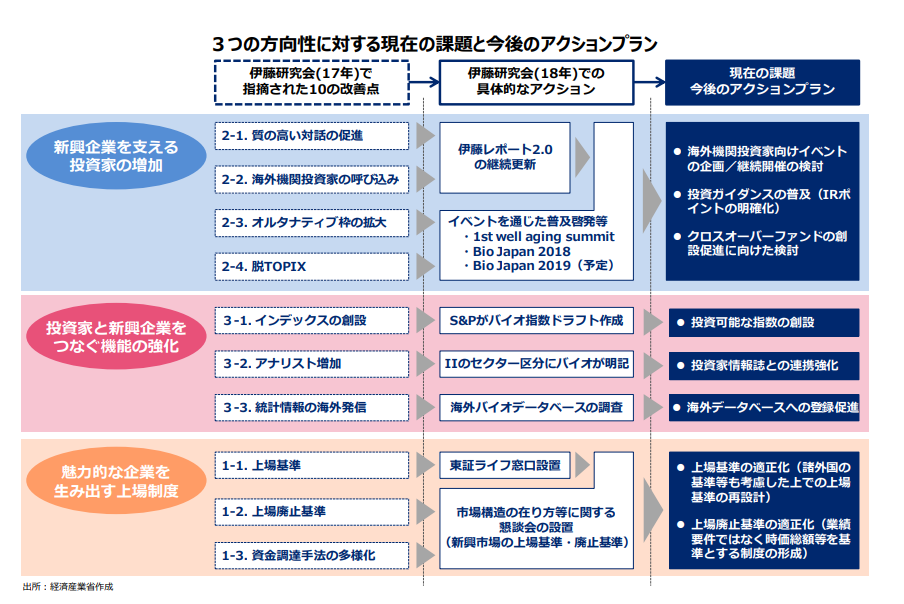
◆Guidebook on Information Disclosure for Encouraging Dialogues between Biotech Venture Businesses and Investors
The "Guidebook on Information Disclosure for Encouraging Dialogues between Biotech Venture Businesses and Investors" was designed to provide a guideline for biotech venture businesses to follow when disclosing especially non-financial information that is deemed necessary from investors' point of view. Details can be viewed here.
"Guidebook on Information Disclosure for Encouraging Dialogues between Biotech Venture Businesses and Investors" (PDF format: 6,253KB)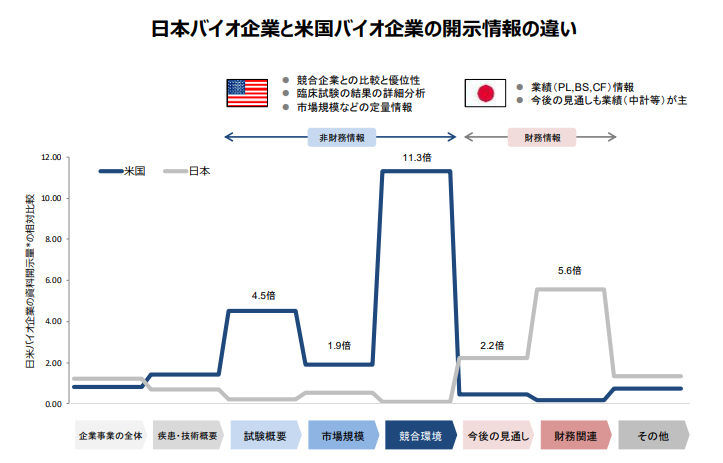
◆Clarifying the listing criteria and reviewing the delisting criteria of Tokyo Stock Exchange
The study group held discussions on clarifying the listing criteria and reviewing the delisting criteria of Tokyo Stock Exchange, aiming to eliminate issues faced by the biotech startup companies immediately before and after becoming listed.
The 8th meeting of the Study Group for Encouraging Dialogue between Biotech Venture Businesses and Investors.Establishing an Environment for the Listing of Biotech Venture Businesses (PDF Format 1,326KB)
Examples of initiative to support startup businesses
◆The Medical Venture/Total Support Business "MEDISO" (Ministry of Health, Labour and Welfare)

The Ministry of Health, Labour and Welfare established a comprehensive portal site for medical venture businesses as part of the "Medical Venture/Total Support Business".
Medical venture businesses can send inquiries on research and development, drug approval, expansion to overseas through the portal site.
*Please contact the Ministry of Health, Labour and Welfare for further details of this initiative.
◆Portal site for the initiatives to support startup businesses

Portal site is now available to access information regarding the startup-related support initiatives undertaken by the Ministry of Economy, Trade and Industry and its relevant Incorporated Administrative Agencies.
For your convenience, this portal site enables searches to discover available initiatives and the information regarding applications according to the preferred support category.
◆Establishment of Task Force for the Creation of New Startup Markets
The Ministry of Economy, Trade and Industry will establish "Task Force for the Creation of New Startup Markets" consisting of expert lawyers to advise on regulations and promote utilization of various regulatory reform systems in order to accelerate creation of new startup markets.
(Reference) System to Remove Gray Zone Areas / "Regulatory Sandbox Scheme" Project / System of Special Arrangements for Corporate Field Test
◆"Mirasapo plus" - A Website for small and medium-sized companies looking for subsidies and general support (The Small and Medium Enterprise Agency)

The website enables searches on support initiatives (systems) for small to medium-sized companies and provides details of each system and information regarding applications.
- Report on the "Key Points in the Management of Pharmaceutical Biotech Startups to Learn from the Specific Cases" (FY2010) (PDF Format: 1,763KB)

- Survey of the Bio Industry (FY2000-FY2010)
Contacts
Bio-Industry Division
Commerce and Service Industry Policy Group
TEL:03-3501-8625 (Direct)
FAX:03-3501-0197
Last updated:2023-03-20